UW researchers will develop gene editing therapy to treat blindness
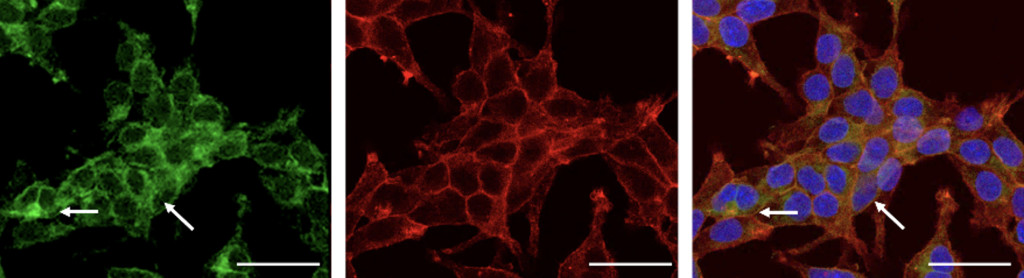
Microscopic images showing the process of fixing inherited mutations within human cells through genome editing. Red and green mark channel proteins inside cells, and blue marks each cell’s nucleus, which houses the cell’s genetic code. Research led by UW–Madison will develop gene-editing drug therapies to treat blindness in humans.
With new support from the National Institutes of Health, a team of researchers at the Wisconsin Institute for Discovery will lead drug therapeutics testing for two diseases known to cause blindness.
Over the next five years, the collaborative project will use the $29 million NIH grant to merge new drug delivery systems with advanced genome CRISPR technology, innovating new treatments for Best Disease (BD) and Leber Congenital Amaurosis (LCA), both of which are currently untreatable hereditary diseases.
“Genetic mutations can cause some of the most rare and devastating disorders of the nervous system,” said Walter Koroshetz, co-chair of the Somatic Cell Genome Editing Program and director of the National Institute of Neurological Disorders and Stroke. “Thanks to large-scale efforts like the SCGP, we are starting to bring tools into the clinic to edit out these gene mutations. While there are still challenges to overcome, the level of hope for effective treatments is high.”
The researchers decided to focus on the eye as their starting point because it is self-contained and isolated from other organs as well as for its accessibility, ease of monitoring and reduced likelihood of adverse immune reactions.

David Gamm
“Our focus is on two different diseases: LCA, a severe and rare group that affects children and their entire vision, and BD which affects older individuals’ central vision and has a slower onset,” says David Gamm, UW–Madison ophthalmology professor and director of the McPherson Eye Research Institute. “By targeting these two diseases, we can gain a broader perspective on the effectiveness of our gene editing therapeutics.”
Krishanu Saha, a professor of biomedical engineering at WID and a member of NIH’s Somatic Cell Genome Editing Consortium, views this grant as a crucial step towards advancing gene editing therapy and drug development on campus.
“The genome editing piece of it is a game changer,” Saha says. “The opportunity to execute it in a safe and meaningful way for patients, specifically Wisconsin patients currently diagnosed with one of these diseases, would be a nice fulfillment of why we do the work and why it’s publicly funded.”

Krishanu Saha
Genome editing involves splicing or cutting DNA at a specific spot, or inserting a DNA template that replaces the cut site. This can correct disease-causing mutations by eliminating or replacing the mutated sequence. Despite significant advancements in CRISPR gene editing technology, it has thus far resulted in few useful drug therapies. This is mainly because although CRISPR can modify the DNA of a single cell, treating billions of cells is necessary for effective treatment.
First, to ensure a therapeutic is safe and effective for patients, a model system is needed to mimic what would happen in a patient, without risking their safety.
“This can be done through animal models or lab-grown cell-based systems,” says Gamm. “Our role is to develop, grow, and maintain the cell-based system for testing.”
Additionally, most CRISPR technology uses a virus delivery system that is currently hindered by unintended off-target effects, such as reduced durability, undesirable immune reactions and supply chain difficulties. To overcome these limitations, the project aims to leverage nanotechnology to develop novel methods for efficient drug delivery of the CRISPR gene editor.

Shaoqin “Sarah” Gong
One delivery approach will be led by Shaoqin “Sarah” Gong, UW–Madison professor of ophthalmology and visual sciences and biomedical engineering.
“Developing a safe and efficient delivery system for the CRISPR genome editor is essential for clinical translation,” says Gong.
Her work focuses on a new family of nanoparticles that can carry genome-editing tools into target organs or cells around the body and then harmlessly dissolve.
In the past, there have been biosafety issues resulting from prolonged expression of gene editors via viral delivery. However, the Gong lab has engineered biodegradable nanoparticles that can deliver genome editors in a way that reduces the off-target editing effects.
Early studies have shown no adverse events in human cell cultures or mouse models. With U19 grant support, the team aims to optimize the nanoparticle formulations for higher editing efficiency, develop a manufacturing process and evaluate biosafety and efficiency in non-human primates. This will lead to a safer and more efficient nanoparticles-based ocular gene editing therapy.
Another approach to improving the delivery of genome editing therapeutics involves a partnership with start-up biotechnology company Spotlight Therapeutics. The California-based company will use a multi-prong approach to solving the delivery challenges using proteins and peptides. They will also focus on streamlining the industry side of developing drug therapeutics, from conceptualization to implementation.
“This project could have a potentially durable impact,” remarks Saha. “Just trying is a big deal. It’s a long road from the design stage of paper and pencil to formulating effective therapeutics with a lifetime impact. It takes lots of investment. The fact that we are piecing together the resources and the people here in Madison makes that really exciting and meaningful.”
Another challenge is one of economics. Rare disorders and diseases are not appealing to industry pharmaceuticals because the market cannot sustain the millions of dollars and time it takes to invest in the resources needed to show genome editing therapeutics are safe and effective.
“This grant offers us the resources to improve processes, develop a safe and effective patient treatment model system and enhance visual function. Although they may not eliminate the disease entirely, the goal is to create meaningful improvement,” says Gamm.
The grant began in May and is one of five multidisciplinary grants that the NIH will award in 2023. UW–Madison will subcontract portions of the funding to three other institutions — the Mordgridge Institute for Research, Spotlight Therapeutics and the University of Massachusetts Medical School — who have groups working on smaller aspects of the project. Principal investigators on the project include Krishanu Saha, Shaoqin Gong, David Gamm, Bikash Pattnaik, Mary Janatpour, William Murphy, Jonathan Watts, Michael Nork, Brian Dattilo and Melissa Skala.



