This protein can shred our cells. Or it can help us think.
A new study reveals that a protein long known to play a role in communication between cells in the brain is also capable of obliterating cells if left unchecked because of its penchant for twisting and puncturing the membranes surrounding cells.
On its own, the protein — known as complexin — is so toxic it can shred cells. Yet, in the brain, a suite of controls makes sure the protein plays nice and helps cells called neurons communicate by aiding in the release of compounds called neurotransmitters.
The findings, published Feb. 7 in Nature Structural and Molecular Biology, emphasize how little we still know about how our brains work. Billions of times every second, the brain’s neurons pass information to one another. While many proteins play a role in this crucial task, just how they accomplish it remains stubbornly mysterious.
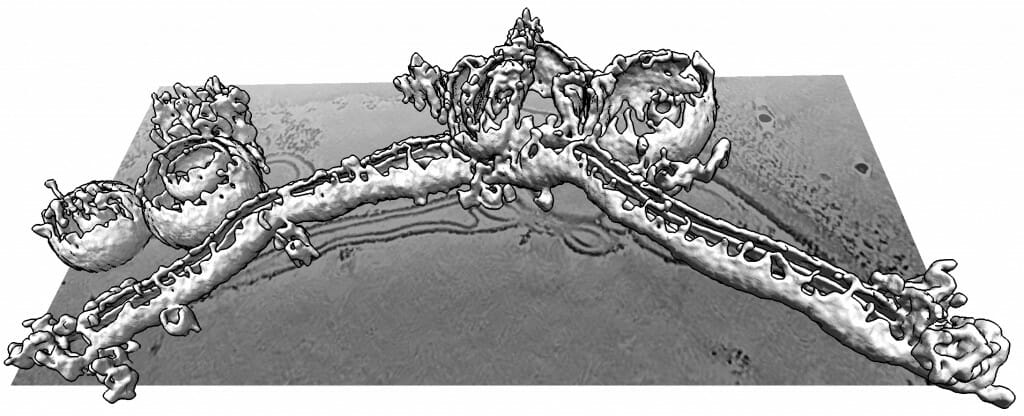
A 3D ultrahigh-resolution image shows how complexin can distort, shred and elongate simulated cellular membranes. Complexin is important for releasing neurotransmitters in the brain, but must be regulated to not damage cells. Courtesy of the Dorit Hanein lab
It all starts inside a neuron when a tiny packet of neurotransmitters fuses with the cell’s outer membrane. That packet then gets released as cargo to make its way to the next neuron.
“We argue that’s the most interesting membrane fusion event in our bodies, because it’s the one that underlies this conversation. It controls remembering and forgetting. It is everything,” says Ed Chapman, professor of neuroscience at the University of Wisconsin School of Medicine and Public Health. “Yet to this day, nobody knows just how the proteins involved in this process really work.”
In trying to better understand these proteins, Chapman’s lab and their collaborators discovered the surprising power of complexin.
“We came to this discovery by accident,” says Kevin Courtney, a Chapman lab postdoctoral fellow who helped lead the new study alongside biophysics graduate student Lanxi Wu.
While setting up experiments to test a collection of different membrane-fusing proteins, a previous researcher in the Chapman lab, Huan Bao, added complexin to a tiny simulation of cell membranes. To his surprise, the membranes went haywire. At first, it looked like the experiment was just a failure.
Bao eventually left to start his own lab, but remaining members of Chapman’s group continued to study complexin. They found that it dramatically bends and reorders membranes. Live videos show the protein pinching off small bubbles of membrane while simultaneously poking holes in them.
Ultrahigh-resolution 3D images produced by the lab of Dorit Hanein and Niels Volkmann at the Scintillon Institute in San Diego also revealed that complexin could form twisting curlicues of broken-apart membranes.
The scientists traced this dramatic ability to a tiny portion of the complexin protein, located at its very tip. Intriguingly, this small chunk closely resembles a variety of other peptides (small bits of protein) that kill bacteria by poking holes in them. One such protein is melittin, the core component of bee venom.
So, the team swapped in melittin at the end of complexin and saw that the complexin-venom hybrid worked just as well as the native form. This swapping experiment demonstrated that complexin achieves its membrane-discombobulating power from being able to insert itself into the membranes, just as the venom does.
Complexin is able to poke holes in simulated cells — allowing a pink dye to light up — while also ripping them into smaller bubbles. Courtesy of the Chapman lab.
If complexin showed off its full strength in the body, it would shred neurons to bits. “Of all the proteins we’ve looked at, complexin is the only one that dramatically transforms membranes all by itself,” says Chapman. “It must be subject to some pretty serious regulation, or else we’d all be dead.”
In further experiments, the team discovered some of the ways neurons might keep complexin in check. Most of all, the number of complexin proteins that cooperate at any one time appears to be strictly limited. Only in high numbers does complexin ravage cells. It may be that in small, controlled quantities, it simply promotes membrane fusion.
Now that they’ve learned more about how complexin works, the team is eager to continue testing the other proteins essential in transporting neurotransmitters. Inspired by their surprising complexin discovery, they’re looking forward to carefully watching for other unexpected findings.
“When you’re in the lab, and you get some surprising experiments, sometimes at first you ignore it, because you can’t explain it or it’s just too weird,” says Chapman. “One of the lessons of this research is to have an open mind.”
This work was supported in part by the National Institutes of Health (grants MH061876, NS097362, P01-GM121203, DP2GM140920, S10-OD012372, P01-GM121203, and R01-AI132378), the national Science Foundation (grant. NSF-DMS1661900 and OCI-1053575.) and the PEW innovation funds 864K625. Chapman is an investigator of the Howard Hughes Medical Institute.