Stem cell scientists clear another hurdle in creating transplant arteries

Jue Zhang, lead author on the paper published in Stem Cell Reports, discusses cell images with Matt Brown, a coauthor on the paper and former postdoctoral researcher at the Morgridge Institute. Morgridge Institute for Research
Cardiovascular disease is a major cause of death worldwide, and treating it isn’t easy. The disease wreaks havoc on patients’ blood vessels and can require complex bypass surgery.
Scientists at the Morgridge Institute for Research at the University of Wisconsin–Madison are working toward a dream of creating artery banks — similar to blood banks common today — with readily-available material to replace diseased arteries during surgery.
The latest work in the lab of Morgridge regenerative biologist James Thomson, also a UW–Madison professor of cell and regenerative biology, puts the science one step closer to that goal.
In a paper published online in Stem Cell Reports on May 9, 2019, the Thomson Lab highlights a better way to grow smooth muscle cells, one of the two cellular building blocks of arteries, from pluripotent stem cells. The work also identifies a potential drug for reducing post-surgical risks in patients who undergo bypass surgery.
“We decided to focus on blood vessels because cardiovascular disease is a major cause of death worldwide,” Thomson says. “In the U.S. for example, heart disease and stroke are the No. 1 and No. 3 killers, respectively. And this work also has implications beyond making vessels for transplantation; it’s sort of a stepping stone to more advanced tissue engineering.”
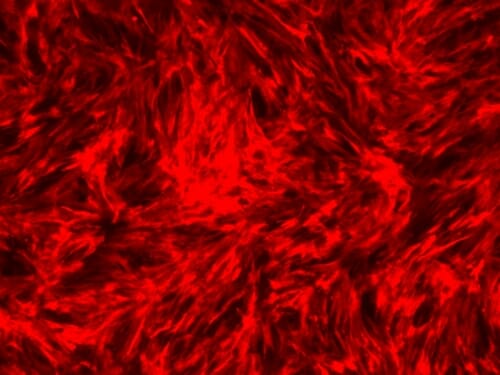
This image depicts live imaging of MYH11-Tom expression in smooth muscle cells differentiated from human pluripotent stem cells using RepSox. MYH11 is a specific protein expressed by smooth muscle cells and is a marker for the mature contractile phenotype. Morgridge Institute for Research
Producing arteries in the lab requires two essential cell types: endothelial cells and smooth muscle cells. In 2017, the lab demonstrated methods to generate and characterize endothelial cells, while the new research focuses on the smooth muscle cells.
Jue Zhang, lead author and a Morgridge associate scientist, says widely used growth factors for producing smooth muscle cells from stem cells can also cause intimal hyperplasia, one of the most common reasons a bypass graft fails.
In intimal hyperplasia, a portion of the arterial wall thickens—due to proliferation and migration of smooth muscle cells—and causes a narrowing of the blood vessel.
“We wanted to have a protocol that can reduce the risk of intimal hyperplasia,”
Zhang says. “It’s a common problem in smooth muscle cell differentiation, and if you want to make a useful artery, you don’t want that risk.”
Healthy smooth muscle cells need the ability to contract, which helps them distribute blood throughout the body and regulate blood pressure. Using a high throughput screen, the team identified a small molecule, known as RepSox, that had the best potential to produce cells with contractile properties.
RepSox was identified out of a screen of 4,804 small molecules. In contrast to current widely used growth factors, RepSox inhibits intimal hyperplasia. It’s more stable than these growth factors and is also a cheaper alternative.
The characteristics that make RepSox good for differentiating smooth muscle cells also make it a desirable drug candidate to reduce risk of post-surgery complications, like intimal hyperplasia, Zhang says. Thus, this stem cell based high throughput screen can be used as a novel strategy for identifying drugs to restrict narrowing of blood vessels.
“Even after you have a bypass surgery, you can have some problems with your artery, like restenosis (narrowing arteries) due to intimal hyperplasia,” Zhang says. “Currently there are only two FDA-approved drugs on the market [to address these problems], and they’re not cell-type specific, meaning they have side effects. We found that RepSox inhibits intimal hyperplasia and has fewer side effects.”
RepSox is cell-type specific, so it inhibits smooth muscle cells and prevents the development of intimal hyperplasia without affecting neighboring cell types like endothelial cells.
While this finding brings scientists closer to improving treatments for cardiovascular disease, Zhang says there’s still another challenge to address: cell maturity.
“Basically this cell type is better than previous efforts, but it’s still not mature yet,” Zhang says. “We need to induce these cells to become more mature, to be more similar to our native artery, to make it more functional.”

Mitch Probasco (left) and Brian McIntosh (right) used lab automation technology to perform a high throughput screen of 4,804 small molecules. In this screen, RepSox was identified as a potent small molecule that improved differentiation of contractile smooth muscle cells from human pluripotent stem cells. Morgridge Institute for Research




