Research to treat placenta could improve human pregnancies
A gene therapy approach to boosting the placenta is safe in monkeys, according to a new short-term study, bringing the potential treatment closer to improving birthweights of human babies and sparing them the complications of an early birth and developmental difficulties later in life.
In humans, placental insufficiency restricts the growth of developing fetuses and typically leads to premature delivery and extended stays in the neonatal intensive care unit.
“The placenta, although transient and typically discarded after pregnancy, is an organ that is so critical to ensuring healthy babies,” says Jenna Schmidt, a scientist who studies placental function, infertility and reproduction at the University of Wisconsin–Madison. “Placental insufficiency contributes to poor nutrient and oxygen transport to the fetus and low birth weight, but there is currently no way to treat the placenta.”
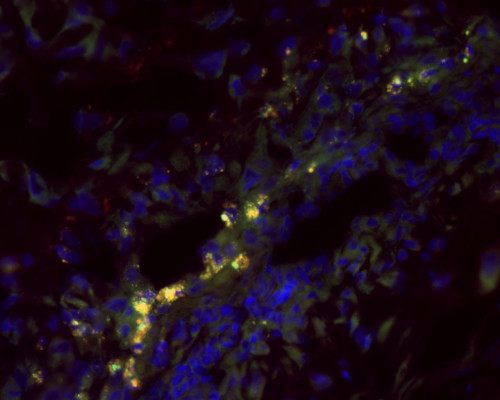
Yellow coloring in this microscope image is the mark of successful delivery of gene therapy via nanoparticles into placental cells from monkeys (each marked by blue-colored nuclei) and expression of a gene expected to boost the fetus-nourishing health of the placenta. Image by Helen Jones/University of Florida
A poor environment in the womb can also lead to problems in adult life, such as cardiovascular disease and neurocognitive developmental conditions, according to Schmidt. Risk factors for placental insufficiency can include high blood pressure, preeclampsia, diabetes and smoking, but in many cases placental insufficiency has no identifiable cause.
Schmidt, a research assistant professor at UW–Madison’s Wisconsin National Primate Research Center, collaborates with University of Florida placenta research expert Helen Jones on strategies for improving the outcomes of pregnancies complicated by placental insufficiency.
“If we can improve placental function to better support growth and development, could we extend those pregnancies to term with the outcome of healthier babies at birth and throughout their lives?” Schmidt asks.
Jones and her lab — with the help of AI platforms to identify targets for treatment — developed a nanoparticle loaded with a small strand of DNA that encodes for a human protein called IGF-1. IGF-1 signaling is important for normal placental development. In pregnancies complicated by fetal growth restriction there are lower levels of this protein, contributing to smaller birthweights and the increased risk of adult diseases.
The researchers injected the nanoparticles into the placentas of pregnant monkeys at the Wisconsin National Primate Research Center, and found that the DNA strands were successfully taken up and expressed in the animals’ placentas within 24 hours without harm to the animals or their developing fetuses and without signs of off-target effects. They published their results recently in the journal Molecular Human Reproduction.
“Our studies so far in mice and guinea pig models of placental insufficiency are very encouraging,” says Jones, whose work is supported by the National Institutes of Health’s Eunice Kennedy Shriver National Institute for Child Health and Development. “And now, with this pilot study demonstrating no detrimental impact in normal non-human primate pregnancies, we are excited to continue to optimize and further target this therapy.”
Jones knew that to move the research toward clinical impact, safety studies were necessary in the rhesus macaque model of human pregnancy.
“This was the first study to test this treatment in macaques and it worked,” Schmidt says. “The transgene was indeed expressed and there was no immune reaction from mom. We saw a signal of the transgene’s expression as far as 10 days after treatment, which was really encouraging. Maybe that could translate into a nanotherapy infusion every two weeks in humans after mid-pregnancy. That is usually when doctors see that the fetus is smaller than normal through ultrasound diagnoses. But there is a lot more work to do before we can move this into human trials.”
The researchers’ next step in rhesus macaques is to extend the therapy through the third trimester of pregnancy, and ultimately to measure the impact on mother and fetus through birth.
“Our goal is to improve placental function, extend pregnancies, and see more healthy babies and adults,” Schmidt says.
The research was supported by the Wisconsin National Primate Research Center Pilot Awards Program through National Institutes of Health grants P51OD011106 and R01HD113327.
Tags: health & medicine, research