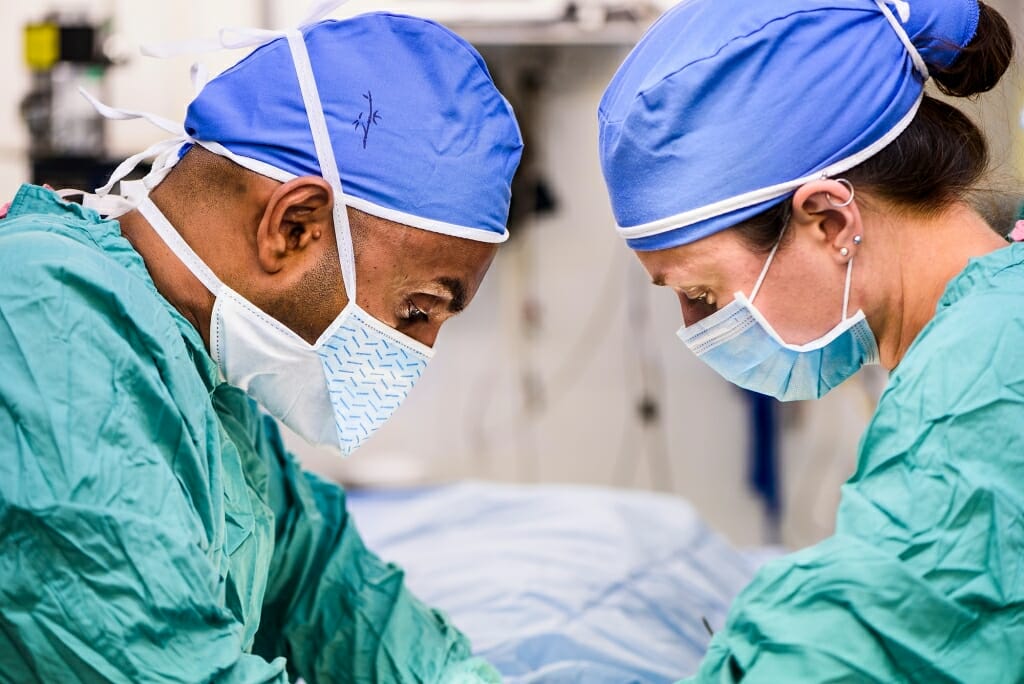
To help kids battling a rare disease, scientists forge a genetic link between people and pigs

Mason Konsitzke, 7, plays in his bedroom at home in Stoughton. Photo: Jeff Miller
Mason Konsitzke is 7. He loves food (especially when he can share it with others) and anything military (both of his grandfathers served). He likes to fly kites and play with his 5-year-old sister, Alexandra. But Mason was born with a disease called neurofibromatosis type 1, or NF1, and each day can present new challenges for him and his family.
NF1 is a genetic disease caused by changes, or mutations, to a single gene in the human DNA library. Roughly one out of 3,000 babies born in the United States have the disease. That’s more than three times as many as have cystic fibrosis. Yet few people have ever heard of NF1.
Mutations in the NF1 gene cause defects in the neurofibromin 1 protein, which acts as a tumor suppressor. Children with NF1 can develop painful tumors along their nerve tracts, including their skin and in their eyes. Sometimes this renders them blind. They are often diagnosed with autism spectrum disorder, though not all children with NF1 are also autistic, and they are sometimes diagnosed with attention deficit hyperactivity disorder. They may have soft bones that bend and break. They are at a higher risk for cancer. And there is no cure.
It was not a disease Mason’s parents, Charles and Malia Konsitzke, had ever heard of. As a newborn, he was healthy. But when Mason was 6 months old, the couple began to suspect something was wrong. Mason developed coffee-and-cream-colored spots all over his body. His father later learned these were a hallmark of NF1. Mason received a genetic diagnosis of the disorder just before his first birthday.
“We were like deer in the headlights,” Malia says. “We were in shock, wondering, what does this mean for us? What does it mean for Mason?”
At 18 months, Mason began to lose his ability to speak. He was falling over, screaming constantly and deliberately banging his head. That’s when an MRI revealed a tumor called a plexiform neurofibroma in a mesh of nerves in the left side of his face. It was growing fast.
A father turns to science
Charles “Chuck” Konsitzke is the associate director of UW–Madison’s Biotechnology Center, a sort of one-stop shop for scientists in need of DNA sequencing, genome editing and other services.
Upon Mason’s diagnosis he began to delve into published NF1 research. He wanted to know where it was happening, who was doing it and how he might be able to help. He sought opinions from experts, wondering how the field could be improved. Many identified the same bottleneck: the lack of a good research model.
In biology, research models are animals, cells, plants, microbes and other living things that allow scientists to study biological processes and recreate diseases in order to better understand them. Good models yield information relevant to humans, but the right model can sometimes be difficult to find.
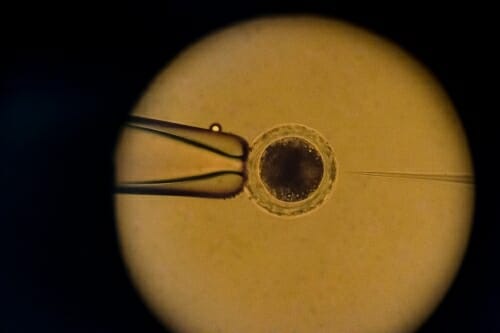
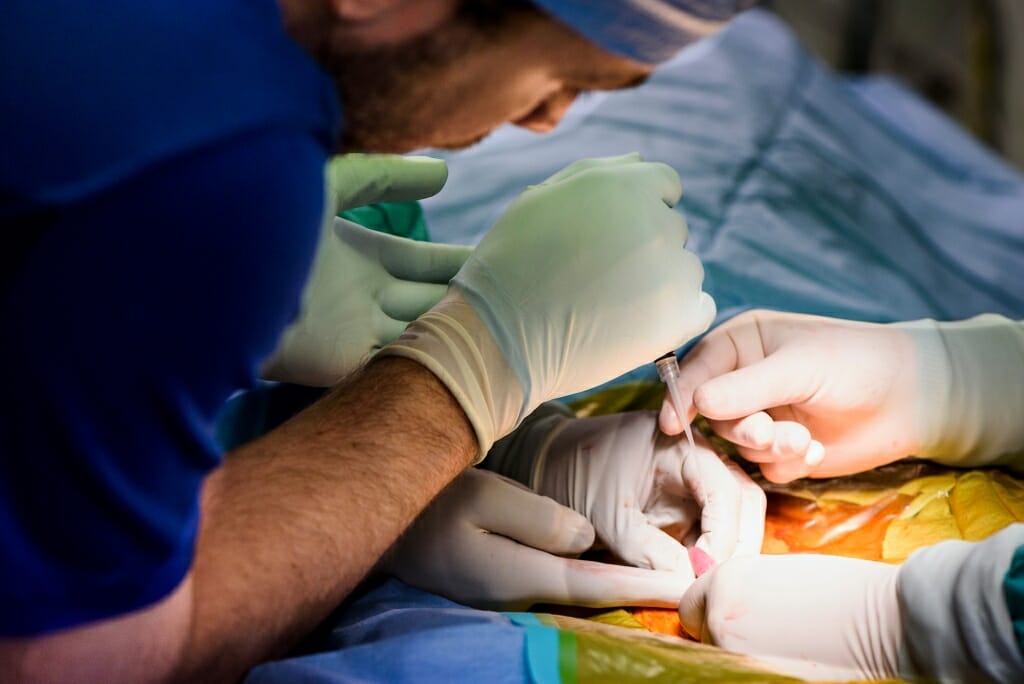
Seen through a microscope, a researcher guides a micro-needle (at right) to inject DNA into a pig embryo at UW–Madison’s Biotechnology Center. Photo: Jeff Miller
NF1 is especially complex, affects many systems of the body and touches many areas of scientific inquiry, from cancer research to neurobiology. Chuck began to search for a better model and in 2013, when Mason was 3, he settled on pigs. Pigs are similar to humans in many ways that other common research animals, such as mice and flies, are not. That includes their size, which means drugs and devices that work on humans can also be tested on pigs. They have a robust immune system, which rodents lack. And they’re intelligent, so scientists can study changes to their cognition.
Chuck then went on the hunt for researchers who studied swine.
Braving the risks
Dhanansayan “Dhanu” Shanmuganayagam, a nutrition and animal sciences professor in the UW–Madison College of Agricultural and Life Sciences, has spent most of his career using swine to study human diseases, particularly heart disease. In fact, he and colleagues in the animal sciences department created the Wisconsin Miniature Swine, a pig that, like people, can develop heart disease under the right conditions.

Dhanansayan “Dhanu” Shanmuganayagam speaks about his NF1 research at a symposium for patients and families at UW–Madison in May. Photo: Jeff Miller
Dhanu’s office was a few blocks from Chuck’s but they’d never met until a few years ago, when they bumped into one another while helping campaign for a new building on campus. They got to know one another and Chuck asked Dhanu whether he had ever heard NF1. He hadn’t. Chuck told him about Mason, about the need for a better model, about the promise that pigs offered to help understand and treat the disease. Would Dhanu join forces to help create that model, Chuck asked.
Dhanu took some time to think about it. He consulted the members of his laboratory. All would be helping to forge this new path. His risks would be their risks. A pig model could fail, leading them all down a blind alley. That kind of outcome can derail a scientist’s entire career.
Dhanu told Chuck he was in.
The risks remain significant, he says, “but I’ve come to terms with it and it’s fine. I’ve been lucky in my career to work on things that have gone to clinic. If it works it’s going to be impactful.”
Meanwhile, Chuck consulted a legal team to ensure he was clear of conflicts of interest, and took steps to ensure his involvement was ethical and not problematic for his staff at the Biotechnology Center.
There aren’t many places in the world where this kind of work – melding basic science with clinical research and a large animal model like swine – is possible. UW–Madison has large biomedical research centers, the capacity for high-powered basic science, and a 1,500-pig research facility called the Swine Research and Teaching Center (SRTC), based in Arlington, a 35-minute drive from campus.
“It’s a brave new frontier, to move into swine,” says David H. Gutmann, a physician and researcher at the Washington University School of Medicine in St. Louis, considered one of the foremost NF1 experts in the world. “I’m glad they’re doing this work at UW–Madison because the combination of specialized resources and expertise are found in very few places worldwide.”
Like scissors for genes
Dhanu and Chuck determined the course they wanted to chart included gene editing, using a powerful new tool known as CRISPR, which stands for Clustered Regularly Interspaced Short Palindromic Repeats.
The genetic technology is reshaping basic biological research. Like a pair of molecular scissors, CRISPR enables scientists to target a stretch of cellular DNA for alteration. They can cut out pieces of DNA or swap out letters in the genome, changing the message it encodes or shutting off genes entirely.
The two set their sights on creating pigs that carry the NF1 mutations they and other researchers are most interested in studying. “But we had to figure out where to start,” says Dhanu. “It’s like learning to fly a space shuttle.”
With Dhanu’s lab manager and lead scientist Jen Meudt at the helm, the team dove in. But the challenges were many.
They had to learn about swine reproduction, about CRISPR and gene editing, how to perform the necessary surgeries on pigs, how to time events so no step of the process failed and ruined all the efforts before it. Again and again, they hit roadblocks.
It took more than a year, but finally, they came up with a plan: The researchers would use artificial insemination to impregnate a female pig carefully primed to produce more eggs than she naturally would. Shortly after fertilization, they would remove the embryos, whisk them to the Biotechnology Center and inject them with a solution containing the gene-editing CRISPR. This would have to be done quickly, while the embryos were still a single cell. When the single cell divided, all the subsequent cells would contain the NF1 mutation. Inject too late and the pig would develop into a mosaic of cells that contain the mutation and those that do not.
Then it would be off to the surrogate mother, a pig chosen to reproductively match the embryo-donating pig. The researchers would perform surgery to implant the CRISPR embryos into her womb. If all went well, months later she would give birth to piglets, at least some of which would carry the desired NF1 mutations.
A few months passed. On November 7, 2016, Chuck and Dhanu were meeting in Madison with a group from the Neurofibromatosis (NF) Network, which supports research and clinical care for NF1. They were sipping coffee when a text came in from Jen: “The mom carrying NF piglets is delivering right now.”
The piglets – eight in all, and four with the NF1 mutation – were a living embodiment of the team’s hard work. They had proved that they could create pigs genetically-engineered to carry the disease. It was an emotional experience for the scientists, involving tears and prayers. They immediately went out to celebrate.
Why are pigs used in research? Learn more
Then they set to work building on that success. One of the four piglets with the mutation was a male. Mason named him Tank. His job is to sire more piglets with the mutation since the changes conferred by CRISPR were designed to be passed on from generation to generation.
The team took the process they’d developed and applied it to other NF1 mutations, including some related to cancer. And they set an even more ambitious goal: precision medicine. A pig personalized for every child with NF1.
With CRISPR, the researchers believe they can take the genetic fingerprint of an individual child’s NF1 mutation and create a pig with that same mutation. They can then test potential medications and treatments and see if they’ll work. Can tumors, like the one that afflicts Mason, be shrunk?
The promise of precision
By the time Mason reached pre-kindergarten, the tumor in his face had grown into his cranial sinus. His parents were told he could lose his sight and his ability to taste.
Surgery wasn’t an option. It was too risky and could leave Mason in even greater pain, permanently. “He’s literally been in pain his whole life,” Malia says.
Then, for reasons doctors couldn’t explain, the tumor stopped progressing. He regained his speech and no longer screamed or struggled to stay upright. His doctors keep a close watch on the tumor with MRI scans. And they continue to work to determine the best medication regimen for the other symptoms that come with his particular variant of NF1. His treatment must be continuously modified. Because of NF1’s unique manifestations, each child is an experiment unto himself.
Pigs develop faster than children do so they offer the possibility of helping predict how NF1 might affect a particular child, enabling parents, doctors, teachers and others to prepare. Earlier intervention for a child who develops autism could lead to better outcomes. Doctors could start working to find drugs to treat tumors before they grow too large.
“Precision medicine is more than matching the right drug to the right gene. With NF1 it’s more complicated and involves searching for the factors that make each individual with NF1 unique,” says Washington University’s David Gutmann. “This is an amazing opportunity to find the risk factors that put an affected child at risk for developing a brain tumor, a bone defect, or another serious complication of NF1.”

Washington University researcher David Gutmann, center, speaks with UW–Madison’s Dhanu Shanmuganayagam, left, and Neha Patel. Photo: Jeff Miller
Dhanu, Chuck and Jen are not doing this work on their own. The team now includes many talented individuals like Biotechnology Center scientists C. Dustin Rubinstein, Kathy Krentz and Michael Sussman, along with Jamie Reichert and his team at the Swine Research and Teaching Center. And there’s now a broader research group, the UW NF1 Translational Research team, that includes Thomas Crenshaw, an animal sciences professor and department chair, and Marc Wolman, a professor of integrative biology.
They have also enlisted the skill and knowledge of Neha Patel, a pediatrician at the University of Wisconsin School of Medicine and Public Health who treats about 150 children with NF1 in Wisconsin and surrounding regions.
Dhanu hopes to make the NF1 pigs accessible to other researchers around the country, charging only what it costs to produce them. And the team plans to use the pigs to help identify metabolic and cellular pathways common to the variety of NF1 mutations, to help target and develop better drugs.
But to accomplish all of this requires funding.
“We’re at a critical moment,” Dhanu says. “We have to turn our successes into funding opportunities.”

Parents and health advocates Lindsay and Ryan Geier participate in a panel discussion during the May event for patients and families. Their daughter Lauren has NF1. Photo: Jeff Miller
The UW NF1 Translational Research team has bootstrapped most of its work so far, relying primarily on funding and donations from the NF Network. Most of that comes from an annual charity golf tournament the Konsitzkes and four other families help organize and run. Called Links for Lauren, the tournament honors Lauren Geier, an 8-year-old girl in Madison with NF1.
Families can play a surprisingly influential role in the fight against rare diseases.“They often provide critical resources and financial support at the earliest stages of a high-risk project, when funding from federal agencies is not possible,” says David Guttman. “Our families, they inspire us because they ask us to do things that are really meaningful and take risks by taking the roads not frequently traveled. Through their involvement they can move the field forward in ways that no one else can.”
Where there’s research, there’s hope
Larry Britzman had no idea there were pigs at UW–Madison that might one day help children like his 12-year-old daughter Mackenzie. He learned that, and much more, in May when he traveled to campus from La Valle, Wisconsin, for a symposium for patients and families.
“I didn’t realize each child is specific,” he says. “I didn’t realize UW has swine research and there aren’t too many facilities in the country researching NF1.”
The NF1 team hopes to host the symposium each year, to invite families to learn more about the science of NF1, to give them a chance to meet researchers and clinicians, and to ask questions and meet other families living with the disease.
“We’ve gone very far in two years because it hasn’t been just about building a model, it’s also been about creating a community around it,” says Dhanu.
The opportunity to work so closely with and on behalf of the people who may ultimately benefit from his work is not something he’d ever experienced. And it’s been profoundly rewarding.
Not long ago, he invited a family whose college-aged daughter has NF1 into his lab. They’d been donors to NF1 causes for years but had never talked to a researcher. “It meant a lot to them and my first thought was: ‘How can we do more of this?’”
He and his lab members now participate in running events like the Madison Half Marathon, often with The NF Team organization, to raise money for NF1 research and to increase awareness. The runners sport neon yellow performance shirts with bold, black lettering. They also participate in the annual charity golf tournament.
“As scientists, we don’t often see the payoff of what we’re working on,” Dhanu says. “It redefines our research priorities and it also aids discovery. The best people to note observations are the people who live with it.”
To him, success can be measured by individuals. “Even if our research just raises awareness and someone gets treated because of what we do, that alone is big,” he says.
Chuck believes the disease is underdiagnosed because very few people are genetically tested for it and most physicians are not familiar with it. So they may diagnose patients with autism or a behavioral disorder and miss the broader picture.
That has frustrated Danielle Wood, a teacher and mother of two who lives in Reedsburg, Wisconsin. Her daughter, Bernadette, is 2 and was diagnosed with NF1 as an infant. Along with springy blonde curls and an arresting smile, Bernadette has a weak abdominal wall, which causes her pain and may require surgery. She wears braces to support her frail ankles.
Danielle, too, has NF1. Her mother had it and so did her grandmother. Though her condition is mild – she simply wears glasses for poor vision caused by a tumor on her optic nerve – deciding whether to have children was hard. Because it is a dominant mutation, Danielle and her husband had at least a 50 percent chance of giving birth to a baby with the disease. Having grown up with NF1, Danielle felt she had a good idea of what to expect. She now sees herself as an advocate for Bernadette.
“While things never move as fast as we want them to, there’s a tremendous amount of exciting progress in this field and where there’s research, there’s hope,” says David Gutmann. UW–Madison is “in a really great position because (it has) young faculty who are excited and a patient community that is challenging them to improve the lives of people with NF1 through research.”
This is what drives Chuck, Dhanu and the rest of the UW NF1 Translational Research team, which is working to establish a NF1 Center for Excellence at UW–Madison. Not only is this possible, David Gutmann says, it is necessary. “There is no established therapy for NF1 and no magic bullet that works for all kids or adults. The challenge for us is to learn more about this disorder so that personalized and effective treatments emerge.”
Moreover, he says, what NF1 teaches researchers will inform their approaches to other conditions. And he’s excited to see what the future holds.
“All of us in the NF field get up every morning and are excited to get to work. What we learn from our colleagues and our families each day brings us one step closer to that better future for children and adults with NF,” he says. “I can imagine getting up every morning and running to work to see what’s happening with those pigs.”
For Mason, the pigs don’t play much of a role in his daily life today. Rather, he relies on regular visits to therapists and other professionals both in and out of school to help him manage his symptoms. He also benefits from the support of his family, from Chuck and Malia to aunts and uncles who have learned all they can about NF1. And the family dog, Donatella, is his packmate, Malia says.
At 7, Mason can still take all that for granted. He can focus on what he loves best, like sharing the tastiest mini pizzas he can make. You should try the pepperoni.

Mason at home with his parents and sister Alexandra, 5. Photo: Jeff Miller