New toolkit helps scientists study natural cell death

Amy Weeks led research to build a more efficient and affordable biochemical toolkit for studying cell death. Photo by Robin Davies
New research from the Weeks Lab in the Department of Biochemistry opens the door for scientists to explore cell death, a critical biochemical process, with greater ease. Here’s the rundown on professor Amy Weeks and her lab’s recent research and paper:
- Cell death is a healthy and constant part of life. Disruptions to the cell death process can result in illness, such as cancerous tumors.
- Available research tools to study the processes and pathways involved in cell death have been expensive and complicated to use.
- Researchers have developed a new toolkit to help scientists study the nuances of cell death using less expensive, off-the-shelf chemicals.
What background info do you need to know?
When deciduous trees drop their leaves each year as the temperatures cool, it is not a sign of organismal death but rather an indication of a healthy tree, full of life. So, too, the orderly and intentional death of cells, or apoptosis, is a sign of good human health.
Cells undergo apoptosis for many different reasons, including eliminating extra immune cells that remain after fighting off an infection or stopping uncontrolled cell division in its tracks.
A cell undergoing apoptosis cuts apart proteins, which are formed from long chains of amino acids called peptides, at specific cleavage sites. The result is new, shorter peptide chains that can stop certain cellular functions or even create potential for new functions. This process of breaking apart proteins, known as proteolysis, is highly regulated. Through proteolysis, a cell’s death is controlled, which helps prevent damage to neighboring cells.
When proteolytic pathways such as apoptosis do not occur as they are supposed to, there can be serious consequences. Cancerous tumors, for example, can form when unhealthy cells fail to undergo apoptosis and instead continue to proliferate.
Why is this process difficult to study?
Scientists lack the full picture about where proteins are cleaved, how cells know where to cut proteins and how each cut alters protein function. That’s because until now, the available tools to study proteolytic pathways were expensive and required specialized expertise to chemically synthesize probes that could recognize cleavage sites. This has limited the tools’ access to many scientists.
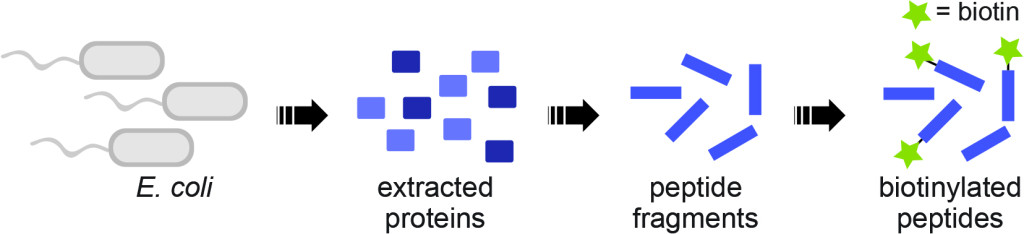
The Weeks Lab used E. coli as a model organism to build a new toolbox for researchers interested in studying cell death and other processes involving proteolytic cleavages. Their process involves linking biotin, which acts as a glue, to newly cut peptide chains in order to attach chemical tools used to isolate the chains. Graphic courtesy of the Weeks lab
How have scientists made progress?
Researchers in the Weeks Lab have developed a new toolbox for scientists studying protein cleavage sites associated with apoptosis and other proteolytic pathways. Taking advantage of the unique biochemical properties of protein fragments, their tool uses less expensive, more efficient, off-the-shelf chemical compounds to help identify sites where proteins were cut.
When a protein is cut, one fragment inherits the original starting point, while the other must establish a new starting point. These new starting points have a uniquely strong affinity for Vitamin B7, also known as biotin. The Weeks Lab used biotin like a glue to attach chemical compounds to the ends of protein fragments, allowing them to isolate the fragments.
From there, the fragments can be analyzed to learn more about how cells identify cleavage sites and how a cut may alter a protein’s function. This information may offer new insight into the impacts on human health when these pathways go awry.
Check out more in the Department of Biochemistry’s series Research in Brief: The What, Why and How of new research exploring the world around us — and inside us.
This research was funded in part by the David and Lucille Packard Fellowship for Science and Engineering (2021-73007) and the Career Award at the Scientific Interface from the Burroughs Wellcome Fund (1017065).
Tags: biochemistry, research



