Morgridge–UW project investigates tissue-engineered arteries for transplant
The prospect of creating artery “banks” available for cardiovascular surgery, bypassing the need to harvest vessels from the patient, could transform treatment of many common heart and vascular ailments.
But it’s a big leap from concept to reality.
The Morgridge Institute for Research and the University of Wisconsin–Madison will address both the engineering and biomedical hurdles in this process through a five year, $8 million project funded by the National Heart, Lung, and Blood Institute (NHLBI).
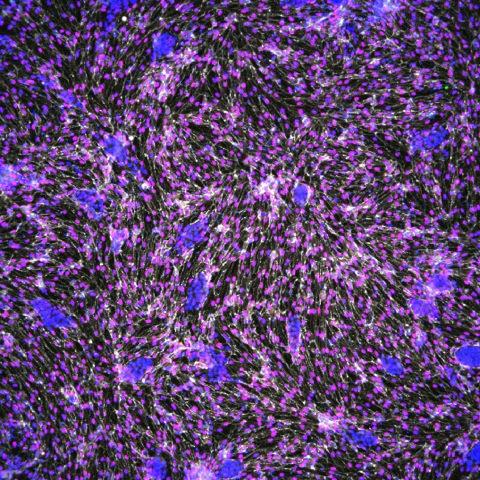
Scientists at the Morgridge Institute for Research and the Wisconsin Institute for Discovery are examining different natural and synthetic materials for their suitability as artery scaffolds. Here, Morgridge scientist Jue Zhang examines a scaffold material produced by a silk spinning machine.
Patients needing bypass surgeries would benefit from a better source for arteries, says project leader James Thomson, a stem cell research pioneer and director of regenerative biology at Morgridge. Replacement tissue currently comes from another part of the patient’s body, and suitable tissue can’t be found for many patients. Current synthetic alternatives also fail at a high rate.
Diseases of blood vessels — including coronary artery disease — kill more people worldwide than any other single cause.
“Tissue engineering for blood vessels is a pretty mature field,” says Thomson. “But there are still two major problems: One is the time it takes to make the vessels, and the other is the source of the cells to grow them.”
For example, taking induced pluripotent (iPS) stem cells from an individual patient, growing the relevant cells and assembling them into an artery would overcome the problem of transplant rejection, Thomson says. However, it would be cost-prohibitive and take months to complete — too long to be clinically useful to a patient.
The promising alternative is to create tissue with cells banked from a unique population of people who are genetically compatible donors, based on rare alleles that circumvent rejection. Alleles are gene pairings that control certain characteristics, such as blood type. It has been estimated that about 100 different cell lines from this rare population would be enough to cover a majority of the U.S. population.
The Morgridge and UW–Madison effort covers four phases and addresses key questions about the feasibility of this approach. The model for the project is designed around treating critical limb ischemia, a debilitating condition that restricts blood flow to limbs and often leads to amputation or death.
The Thomson group is working to create the optimal cellular building blocks of the artery — endothelial and smooth muscle cells — that will be most suitable for transplantation and continue to grow and remodel in the patient.
In tandem, a team led by UW–Madison engineer Tom Turng will develop scaffolds from natural and synthetic materials to provide structure and shape for the artery. UW–Madison engineer Naomi Chesler will build a bioreactor that provides an environment in which the arterial cells can grow around the scaffolding.
The transplant surgery and resulting immune response will then be tested using a monkey limb ischemia model at the Wisconsin National Primate Research Center. Having a primate model is important to produce results more relevant to human health than those from mice or other short-lived animals. Pathology and laboratory medicine Professor Igor Slukvin is leading the primate center effort.
Finally, the UW–Madison Waisman Biomanufacturing facility will lead the production of arterial cells that meet FDA standards for human clinical trials, paving the way for potential treatments for limb ischemia in humans.
If the entire process works, Thomson estimates that potential human therapies remain about 10 years away.
“This is a collaboration that really highlights what the Morgridge Institute does well because we are able to bring together multiple investigators from different departments and centers around campus,” Thomson says. “We have an unusual combination of resources in Madison to be able to pull this off.”
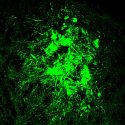
Endothilial cells engineered from iPS stem cells are essential building blocks in the vascular engineering project. Jue Zhang, assistant scientist, Morgridge Institute for Research
The heart of the partnership to date has been between Thomson’s team and the BIONATES program in the Wisconsin Institute for Discovery, led by Turng. Both labs operate on the same floor of the Discovery Building, and the three-year collaboration is a prime example of how the public and private institutes work together to foster innovation.
As Turng works to determine the optimal choice of materials and methods for construction of the blood vessel scaffolds — biopolymers versus synthetic polymers, or a combination of both — the partnership inside the Discovery Building is vital to progress. “We have direct feedback with Thomson and the other researchers, and the iterative process can be facilitated,” says Turng. “We can speak the same language.”
Providing proof of concept on the blood vessel work should benefit other forms of stem cell therapy, Thomson says.
“This has implications beyond making vessels for transplantation. It’s sort of the stepping stone to more advanced tissue engineering,” he says. “Any form of cellular transplant therapy is going to need a blood supply, and we need to learn how to engineer that blood supply to work with more complex tissues.”