Gene therapy protects against motor neuron disease in rats
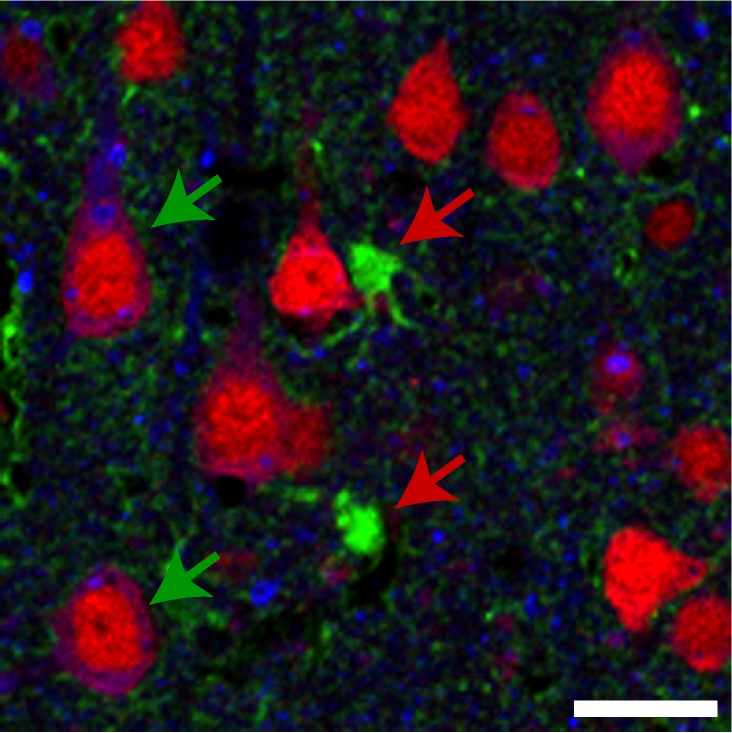
This image shows a section of brain tissue from a rat that received a gene therapy targeting the Trk-fused gene. A mutation in that gene is implicated in the development of certain motor neuron diseases. Green arrows highlight neurons expressing elevated levels of the gene following the therapy. Red arrows indicate other nerve cells called astrocytes that lack increased expression. Image courtesy of Anjon Audhya/UW–Madison
University of Wisconsin–Madison researchers targeting a group of hereditary neurodegenerative diseases have found success using a gene therapy treatment in an animal model. The approach, which uses CRISPR-Cas9 genome editing technology, offers a unique and promising strategy that could one day treat rare but debilitating motor neuron diseases in humans.
Hereditary spastic paraplegia, or HSP, is a group of movement disorders that cause progressive weakness and stiffness in the legs of people with certain inherited genetic mutations. The rare disorders usually lead to physical limitations and use of a wheelchair.
Studying disease processes in animal models is an indispensable part of developing and testing any new treatments before they’re offered to humans. But scientists had historically struggled to replicate HSP’s symptoms and disease progression in animal models.

Anjon Audhya
That changed in 2022, when a group of UW–Madison scientists led by Anjon Audhya, a professor of biomolecular chemistry, used CRISPR-Cas9 technology to develop a rat model that carries a genetic mutation associated with HSP.
The mutation is in the Trk-fused gene, which typically facilitates the transportation of proteins within nerve cells called neurons. When that function is disrupted in people — and rats — it leads to worsening symptoms of weakness and stiffness.
Since 2022, Audhya and his colleagues have refined their rat model and begun testing therapeutic approaches for HSP. They recently developed a strategy that protects rats that carry a genetic mutation causing HSP from developing symptoms.
That approach relies on a genetically engineered virus that targets neurons and introduces a normal version of the Trk-fused gene (which doesn’t include disease-causing mutations) to compensate for the mutated one. The scientists injected this engineered virus into the brains of day-old rats.
“Those animals never got disease,” says Audhya. “So they were able to live for many, many additional weeks, never showing signs of disease. It’s a real demonstration that the gene therapy approach is highly effective in addressing disease symptomology.”
Specifically, the gene therapy approach allowed the non-mutated gene to be expressed in neurons and better support the transportation of proteins, preventing disease. The group recently reported their findings in the journal Proceedings of the National Academy of Sciences.
Additionally, the researchers made a fundamental discovery about HSP — it’s primarily a disorder of neurons, rather than other cells found in the brain. When the team tested a similar strategy on cells called astrocytes, those animals still developed disease.
Audhya and his colleagues are already moving on to follow-up studies developing another animal model and targeting a different gene mutation, which is more prevalent in HSP patients. They’re also planning to inject therapeutics via the spinal cord, which is closer to how treatment in humans would occur.
Because HSP is a rare disease, funding for these studies can be difficult to secure. Audhya notes the support his team has received from Blu Genes Foundation, The Lilly and Blair Foundation and CureSPG4 Foundation has been a crucial part of their progress toward a potential cure for HSP.
“We ultimately hope our preclinical gene therapy efforts will lead to a new clinical trial in patients in the years to come,” he says.
Additional funding to support this project came from the National Institutes of Health (R01NS124165 and R35GM134865).



