Cellular communication system in mice helps control female fertility
When Joan Jorgensen was an undergraduate at the University of Wisconsin–Madison, her roommate confided that she had just one period before going through menopause in high school. Doctors told Jorgensen’s roommate that she would never have biological children.
“This is devastating news at any age, let alone a high school girl,” says Jorgensen, who is now a professor in the Department of Comparative Biosciences at the UW–Madison School of Veterinary Medicine.
That experience stuck with Jorgensen, whose research focuses on fertility problems like premature ovarian failure, which leads to an early loss of viable eggs and which her roommate experienced. Using animal models, Jorgensen tries to understand how female fertility is affected by development of the ovary, which includes how cells organize to support eggs for the entire lifetime of that individual.
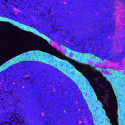
In new research published Aug. 2 in the journal PLOS Genetics, Jorgensen, graduate researcher Anqi Fu and others discovered that two genes work together to construct a cellular communication system in the ovaries of mice to maintain healthy eggs. The researchers describe this system as a series of junctions between the eggs and the cells that surround and support the eggs, known as granulosa cells. Both cells reach out to form multiple junctions that exchange information and ensure the proper development and survival of the egg leading up to ovulation.
This research provides a piece of the puzzle of female infertility, and Jorgensen looks to build off these findings to uncover more information on premature ovarian failure and other fertility problems. Jorgensen and Fu collaborated with researchers at the University of Melbourne, Monash University, and the University of Toronto to complete this work.
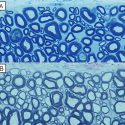
Premature ovarian failure, in which the ovaries stop producing estrogen, is often caused by premature loss of the egg supply and affects as many as 3 percent of all women, according to the National Institutes of Health. In most cases the cause is unknown. Problems with the development of follicles — the combination of an egg and its surrounding granulosa cells — are likely behind many cases of premature ovarian failure.
Jorgensen’s lab had previously found that mice missing two genes, IRX3 and IRX5, had defective follicles. In the current study, they looked for how these genes work together to keep follicles healthy.
The researchers showed that mice with either IRX3 or IRX5 deleted had fewer pups, which led the researchers to suspect that communication within the follicle was breaking down. Looking within the ovary, they tracked the expression of each gene.
Early on, the researchers saw that IRX3 and IRX5 were expressed throughout the follicle. But as the follicle began to mature, IRX3 became isolated to the egg, while IRX5 was only expressed in the granulosa cells.
From their separate vantage points, these two genes synchronize the two cell types to help them establish communication networks. Jorgensen’s team saw that the granulosa cells and the eggs extend parts of their membranes to form junctions with each other. These junctions allow signals to be transported in both directions. With IRX3 or IRX5 deleted, these junctions fell apart, interrupting communication within the follicle and destabilizing it.
“We think of IRX3 and IRX5 as the supervisors in connecting these two cells,” says Jorgensen.
Despite this discovery of a role for these genes in follicle development in mice, researchers still aren’t sure if these same genes have a similar effect in humans.
“That’s another thing we would like to learn — we want to be able to link it to human causes,” says Jorgensen.
Jorgensen and Fu say the next step will be to evaluate exactly how these genes direct these key cell-to-cell interactions.
“If we can figure out how those networks are placed, we think that will be a major step in understanding the basic foundations of how follicles are built,” says Jorgensen. “That will go a long way towards helping women that have infertility, especially those that undergo premature ovarian failure.”
This work was supported by National Institutes of Health (R01HD075079), National Cancer Institute (P30 CA014520), and a University of Wisconsin Carbone Cancer Center Support Grant.