Antibody targets key cancer marker; opens door to better diagnosis, therapy
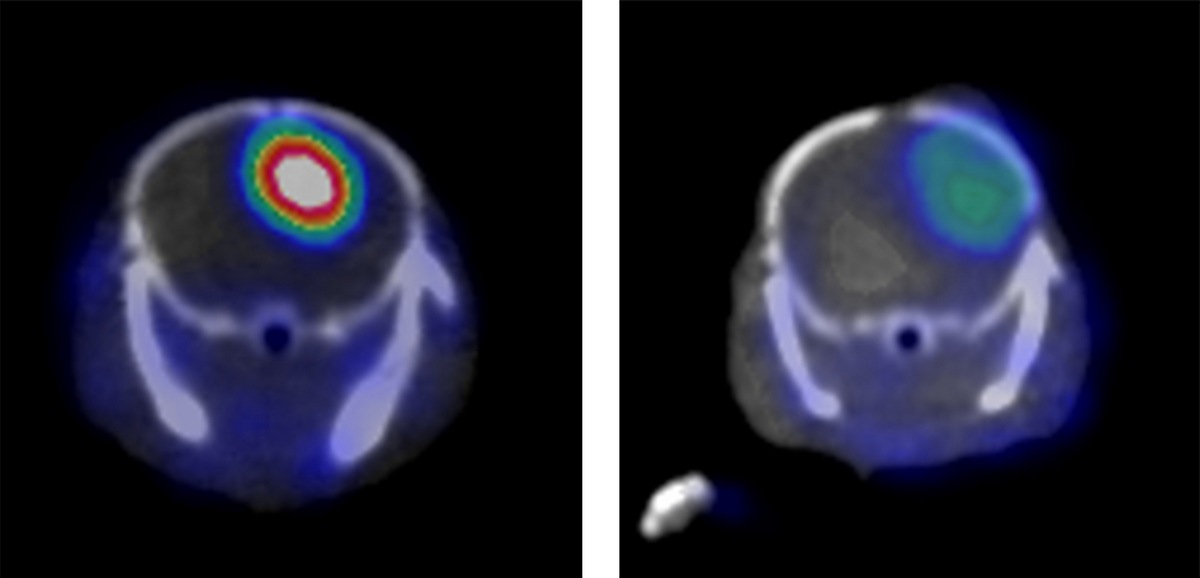
Researchers guided by Weibo Cai have identified a molecular marker on aggressive forms of the brain cancer glioblastoma multiforme and created an antibody that latches onto it. When the antibody is linked to a label that shows up in a PET scanner, an aggressive cancer in a mouse model shines brightly (left). A similar cancer without the molecular marker (right) is much less obvious in the PET scan.
Image: Weibo Cai
University of Wisconsin–Madison researchers have created a molecular structure that attaches to a molecule on highly aggressive brain cancer and causes tumors to light up in a scanning machine. In mouse models of human brain cancer, their tag is easily seen in a PET scanner, which is commonly used to detect cancer.
The study, reported in the journal PNAS the week of Nov. 9, began by identifying a marker for the deadly cancer glioblastoma multiforme in a database of cancer genomes that amasses genetic sequences from labs around the world. In that atlas, Weibo Cai, associate professor of radiology and medical physics, noticed a gene called CD146 that is highly active in glioblastoma. “When we looked for a potential target, CD146 seemed ideal, as it was correlated with poor outcome,” says Cai.

Weibo Cai
The CD146 gene places a unique protein, also called CD146, on the surface of cells.
Cai’s strategy focused on an antibody that can recognize the CD146 protein. Antibodies are complexly shaped molecules that can attach to specific proteins. In biology, antibodies transmit information across a cell membrane to stimulate or silence various processes. In medicine, they are used to identify, alter or kill cells having the correct protein on the surface.
After Cai’s group created an antibody that selectively links to the CD146 protein, they joined it to a copper isotope that is easily seen in a PET scanner. Then they implanted a human glioblastoma sample into a mouse, injected the antibody-marker combination into the blood, and waited for the antibody to spread through the animal.
When the mouse was placed in a PET scanner, the instrument identified tumors with a high level of CD146 protein on the outside of their cells. The signal from a genetically distinct glioblastoma with low CD146 activity was much weaker.
Identifying cancer at the cellular level is a chief treatment goal, as most cancers kill when cancer cells metastasize and “seed” tumors in new locations. Preliminary data from Cai’s lab suggests that the CD146 marker is associated with cancer stem cells — primitive tumor cells that are considered key in metastasis.
Beyond that, the research could go in several directions, Cai says. “We’ve created a tag that — at least in our mouse model — is highly specific for this aggressive brain cancer. If the technique proves out in further tests, it could be used to diagnose some strains of aggressive glioblastoma, and also to evaluate treatment progress or even to test potential drugs.”
“These results could have far-ranging implications for improving outcomes in cancers for which there is currently much need for improvement.”
Peter Choyke
Because CD146 also appears on some ovarian, liver and lung tumors, the technique could have a broader reach. “Our tests with other tumor types confirm an association between the level of CD146 activity and the aggressiveness of the tumor,” Cai says.
“This targets tumors with the worst survival,” Cai adds, “but I want to emphasize that human trials are some years in the future.”
“Because this biomarker occurs in several other aggressive cancers, and is implicated in specific cellular processes that we know play a role in metastasis, these results could have far-ranging implications for improving outcomes in cancers for which there is currently much need for improvement,” says Peter Choyke, director of the Molecular Imaging Program at the National Cancer Institute.
“Their noninvasive test for this biomarker could be helpful in many ways, including surgical removal, detection of recurrences and monitoring of therapy,” Choyke adds. “Because this approach only applies to an identifiable subset of this brain cancer, I see it as another step toward the goal of personalized medicine.”
The study also demonstrated an improved system for creating antibodies that is “faster, cheaper and easier than all previous methods,” says Cai. “Most methods generate antibodies that recognize short fragments of the target protein, whereas our antibody recognizes the target in the biologically active form. So the antibodies can be readily used in living subjects, such as the animal models in this study and potentially in patients.”
The research team included Reinier Hernandez, Cai’s Ph.D. candidate in Madison, and Beth Meyerand, chair of biomedical engineering at UW–Madison. First author Yunan Yang received a Ph.D. with Cai and recently formed a company, Oligene LLC, to develop a pipeline of antibodies for clinical studies in the early diagnosis and treatment of tumors.
Tags: animal research, cancer, health & medicine, research



