Essential mechanism of symbiosis found in Hawaiian squid

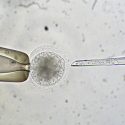
The Hawaiian bobtail squid, in the lab of Margaret McFall-Ngai, offers a unique platform for studying the symbiotic interaction between an animal and bacteria it needs to survive.
Photo: Chris Frazee, UW School of Medicine and Public Health
Experiments at UW–Madison with a small squid that glows in the dark have uncovered a complex conversation that allows the newly hatched squid to attract the glowing, symbiotic bacteria that disguises it against predators.
Increasingly, scientists recognize that such symbiosis plays a critical role in many biological processes associated with human health and disease, says senior author Margaret McFall-Ngai, a professor of medical microbiology and immunology at UW–Madison.
The dialogue begins when fewer than five bacterial cells latch onto the squid. After the animal senses the bacteria, the squid responds in two ways: increasing breakdown of a sugar that eventually guides the bacteria toward their home in the squid’s light-emitting organ, and producing antimicrobial compounds that deter other types of bacteria.
Margaret McFall-Ngai
The squid are native to the ocean around Hawaii. During the day, they burrow into the ocean floor for protection. At night, while they forage, they appear as shadows to predators looking up against the bright ocean surface. But when the bacteria light up, the squid are camouflaged.
During decades of study, McFall-Ngai has been impressed by the specificity of the relationship: The squid are born sterile, but within hours, in water containing thousands of types of bacteria, they select only the one they need, then give it a home, in return for that protective light.
The question of how the animal chooses among those thousands of other bacterial cells has great significance, McFall-Ngai says, as a much more complicated version of this same selection occurs after a human birth. “It’s remarkable, when humans are born, they are not associated with a random bacterial sample of their environment. We have to choose the correct microbes.”
In a study published today in Cell Host & Microbe, McFall-Ngai and first author Natacha Kremer, an honorary associate fellow in medical microbiology at the University of Lyon, France, report that the first few bacteria, after attaching to the squid, signal broad changes in the squid’s gene expression. “We were interested in how the squid responds specifically to the correct bacteria, and how this happens very early in the initial contact,” says Caitlin Brennan, a co-author who is a post-doctoral fellow in the lab of Ned Ruby, a professor of medical microbiology at UW–Madison.
Increasingly, scientists recognize that such symbiosis plays a critical role in many biological processes associated with human health and disease.
After signaling to the squid, the bacteria wait a few hours before moving into niches in the squid’s light organ, where they will multiply and glow at night, protecting the animal against predators. During this wait, the squid releases an enzyme to convert chitin, a component of its mucus, into a sugar that serves as an attractant for the bacteria. After this initial chemical conversation, the bacteria become responsive to the squid’s “come-hither” signal, and they move inside the squid. At the same time, a second widespread change in gene activity causes the squid to make that antimicrobial agent.
“We find that the bacteria require priming; they won’t swim toward the chemical signal unless they have seen it beforehand, while first attached to the squid. The bacteria are responding to their environment, which the host is changing after sensing the bacteria. We’re watching a real conversation between the host and the symbiont,” says Brennan.
Virtually all higher organisms coexist with bacteria, and after hatching or birth most or all of them need to be “infected” with the microbes they need for normal functioning. People, for example, “have very specific microbiota at each region of the gut,” McFall-Ngai says. “We can have about 700 types in the mouth, 100 in the stomach, and 1,000 in the large intestine.”
For a century, scientists have used simple organisms — fruit flies, yeast, and nematodes — to learn the rules of the biological road. The squid offers an ultra-simple version for studies of symbiosis, McFall-Ngai says, “where you have a hope of figuring out the dialogue between partners because there are only two species.”
“When biologists are trying to understand a conversation between a microbe and a human host, it’s like walking into the biggest cocktail party and trying to figure out what is going on in all the conversations.”
Margaret McFall-Ngai
All animals descended from aquatic ancestors, McFall-Ngai points out, and so we all share many systems for chemical communication with our microbial companions. “While animals evolved in the marine environment, they were under tremendous selection pressure from the microbes in the seawater. If you look at one element of that association, how bacteria talk to animal cells, you’ll see that conserved all the way through the animals.”
And as scientists find a growing role — both helpful and harmful — for these microbes in areas like immunity, cancer and obesity, it all starts with a conversation, McFall-Ngai says. “When biologists are trying to understand a conversation between a microbe and a human host, it’s like walking into the biggest cocktail party and trying to figure out what is going on in all the conversations. With the squid, it’s like “My Dinner With André.” You can understand it; it’s a give and take that can be deciphered.”