In chemical reactions, water adds speed without heat
An international team of researchers has discovered how adding trace amounts of water can tremendously speed up chemical reactions-such as hydrogenation and hydrogenolysis-in which hydrogen is one of the reactants, or starting materials.
Led by Manos Mavrikakis, the Paul A. Elfers professor of chemical and biological engineering at the University of Wisconsin–Madison, and Flemming Besenbacher, a professor of physics and astronomy at the University of Aarhus, Denmark, the team published its findings in the May 18 issue of the journal Science.
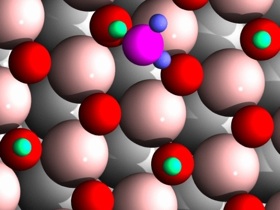
Through an interaction with hydrogen atoms (green), a water molecule (magenta and blue) moves rapidly across a metal oxide surface. This atomic-scale speed leads to more efficient chemical reactions.
Hydrogenation and hydrogenolysis reactions have huge applications in many key industrial sectors, including the petrochemical, pharmaceutical, food and agricultural industries. “In the petrochemical industry, for example, upgrading of oil to gasoline, and in making various biomass-derived products, you need to hydrogenate molecules-to add hydrogen-and all this happens through catalytic transformations,” says Mavrikakis, who is among the top 100 chemists of the 2000-10 decade, according to Thomson Reuters.
A chemical reaction transforms a set of molecules (the reactants) into another set of molecules (the products), and a catalyst is a substance that accelerates that chemical reaction, while not itself being consumed in the process.
In industrial applications, the speed of catalytic transformations is important, says Mavrikakis. “The rate at which the hydrogen atoms diffuse on the surfaces of the catalyst determines, to a large extent, the rate of the chemical reaction-the rate at which we produce the products we want to produce,” he says.
While many researchers have observed that water can accelerate chemical reactions in which hydrogen is a reactant or a product, until now, they lacked a fundamental grasp of how that effect was taking place, says Mavrikakis. “Nobody had appreciated the importance of water, even at the parts per million level,” he says.
In their research, Mavrikakis and Besenbacher drew on their respective theoretical and experimental expertise to study metal oxides, a class of materials often used as catalysts or catalyst supports. They found that the presence of even the most minute amounts of water-on the order of those in an outer-space vacuum-can accelerate the diffusion of hydrogen atoms on iron oxide by 16 orders of magnitude at room temperature.
In other words, water makes hydrogen diffuse 10,000 trillion times faster on metal oxides than it would have diffused in the absence of water. Without water, heat is needed to speed up that motion.
Besenbacher and his colleagues have one of the world’s fastest scanning tunneling microscopes, which has atomic-scale resolution. With it, they could see how quickly hydrogen atoms diffused across iron oxide in the presence of water.
To explain the fundamental mechanisms of how that happened, Mavrikakis and his team used quantum mechanics, a branch of physics that explains the behavior of matter on the atomic scale; and massively parallel computing.
Essentially, when water is present, hydrogen diffuses via a proton transfer, or proton “hopping,” mechanism, in which hydrogen atoms from the oxide surface jump onto nearby water molecules and make hydronium ions, which then deliver their extra proton to the oxide surface and liberate a water molecule. That repeated process leads to rapid hydrogen atom diffusion on the oxide surface.
It’s a process that doesn’t happen willy-nilly, either.
The researchers also showed that when they roll out the proverbial red carpet — a nanoscale “path” templated with hydrogen atoms-on iron oxide, the water will find that path, stay on it, and keep moving. The discovery could be relevant in nanoscale precision applications mediated by water, such as nanofluidics, nanotube sensors, and transfer across biological membranes, among others.
The U.S. Department of Energy Office of Basic Energy Sciences funded the UW–Madison research. Other UW–Madison authors on the Science paper include chemical and biological engineering research scientist Guowen Peng, PhD student Carrie Farberow, and Ph.D. alumnus Lars Grabow (now an assistant professor at the University of Houston). Other authors include Lindsay Merte, Ralf Bechstein, Felix Rieboldt, Wilhelmine Kudernatsch, Stefan Wendt and Erik Laegsgaard of Aarhus University.
